Biological importance of protein glycosylation
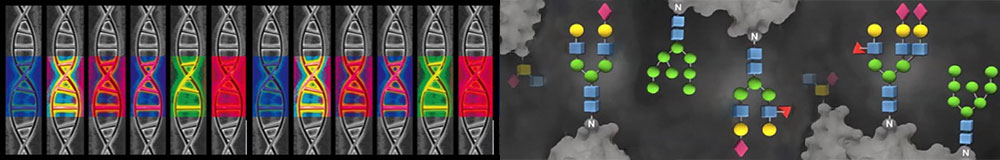
Most integral membrane and extracellular proteins are modified by covalent addition of complex oligosaccharides (glycans), which considerably affects protein structure and function. Glycans and glycan-binding proteins have significant impact on fundamental biological processes such as cell signaling, leukocyte trafficking, innate immunity, and cell-cell interactions. Glycans are assembled from monosaccharide residues through carefully regulated enzyme-directed biosynthetic pathways. In contrast to polypeptides, which are defined by the sequence of nucleotides in the corresponding genes, glycans are shaped by complex dynamic interactions between hundreds of enzymes (encoded by the so-called glyco-genes), transcription factors, ion channels and other proteins (encoded by the glycosylation related genes) (Figure 1). A single genetic or epigenetic change may have an effect on glycan phenotype through change in expression level of these genes (Figure 2).
Since glycans integrate genetic background and environmental factors, they are closely associated with disease and are indeed directly involved in the pathophysiology of virtually every complex disease.
We are interested how protein glycosylation is regulated. Our research in epigenetics of protein glycosylation is guided by two premises:
- epigenetic regulation of protein glycosylation is an important source of glycan variability in a population which could serve as adaptory mechanism (Figure 3) (Lauc, Vojta & Zoldoš 2014; Vojta & Zoldoš 2013);
- epigenetic deregulation of glyco-genes results in aberrant protein glycosylation and this can be a road to a disease (Vojta et al. 2016).
Figure 1. Dozens of glyco-genes and glycosylation related genes are involved in the complex process of protein glycosylation, which takes is taking place in ER and Golgi apparatus. Glycoproteins are then transported either to the cell membrane or secreted from the cell (From: Krištić, Zoldoš & Lauc 2015. Glycoscience: Biology and Medicine).
Figure 2. Glycans integrate activity of dozens of genes: a single nucleotide polymorphisms (SNP) or an epigenetic change in any of these genes may have an effect on a final glycan structure through change in enzyme activity. Glycans are sensitive to environmental stimuli (external or intrinsic) and epigenetic mechanisms (DNA methylation and histone modifications) are mediators between the environment and a glycan phenotype.
Figure 3. Adaptation to changes in the environment through variant glyco-phenotypes is achieved by epigenetic regulation of glyco-genes and glycosylation related genes. Epigenetic mechanisms, such as DNA methylation, histone marks or action of small non-coding RNAs, are mediators between the environment and glyco phenotypes. Transcriptional status of genes involved in glycosylation can change in response to environmental cues in order to achieve appropriate functional change in protein glycosylation, which can become adaptive through long-term epigenetic effects, and can even be transmitted to the next generations.